Cyclophilin A Enzyme
Using an ALCC award, Pratul Agarwal made a discovery that may ultimately lead to drugs with fewer side effects, less
expensive biofuels, and more. "Our discovery is allowing us to perhaps find the knobs that we can use to improve the
catalytic rate of enzymes and perform a host of functions more efficiently," said Agarwal, "The importance of the
structure of enzymes has been known for more than 100 years, but only recently have we started to understand that the
internal motions may be the missing piece of the puzzle to understand how enzymes work," Agarwal said. "If we think
of the tree as the model, the protein moves at the molecular level with the side-chain and residues being the leaves
and the protein backbone being the entire stem." The paper is available for review at the following link:
http://www.plosbiology.org/article/info%3Adoi%2F10.1371%2Fjournal.pbio.1001193
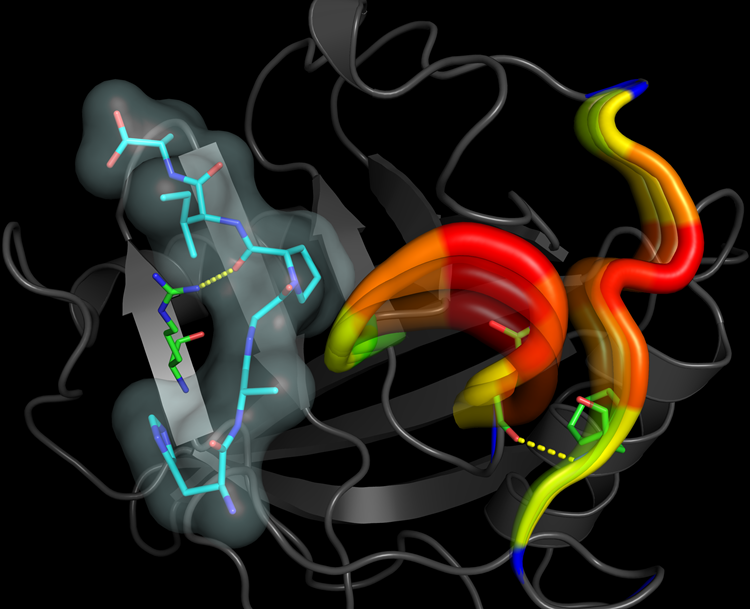
The image below provides a representation for the internal motions coupled to the catalytic step of the enzyme Cyclophilin A. The substrate bound at the active site is shown in cyan sticks and the highly flexible regions in the enzyme are highlighted in a tube-like representation. The transparent tubes indicate the directionality of the motion. The colors on the tube indicate the extent to which these regions move, with red and blue regions representing maximum and minimum mobility, respectively. Hydrogen bond interactions from the surface of the enzyme connect all the way to the active site and are indicated as yellow dashes. The interactions and the internal motions in Cyclophilin A are conserved from bacteria to humans.