Out of Thin Air
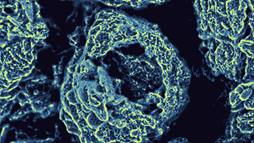 Photo courtesy of Pacific Northwest National Laboratory
Photo courtesy of Pacific Northwest National Laboratory
Microsized pores and tunnels in the electrode facilitated the free flow of oxygen molecules, while nanosized pores provided excellent sites for the lithium-oxygen reactions.
Improvements in battery technology have in some respects played almost as conspicuous a role in the emergence of the contemporary digital age as have advances in microchips—especially when it comes to consumer goods. The portable digital devices that pervade our lives today—smartphones, tablets, e-book readers, GPS receivers, laptops, etc.—virtually all rely on lithium-ion (Li-ion) battery technology born of basic and applied research conducted over the past several decades (a lot of it supported by the U.S. Department of Energy [DOE]). And Li-ion batteries are also of course increasingly the technology of choice for today's hybrid electric vehicles.
Work on Li-ion batteries continues, and there remains room for further progress in the technology. But advanced battery research today is beginning to look beyond the Li-ion battery to fundamentally new battery architectures and chemistries. That's because any comprehensive effort to move beyond today's overwhelming dependence on petroleum and other fossil fuels will almost certainly require not just incremental, but revolutionary advances in batteries and energy storage.
Vastly improved batteries and energy storage will hold the key to integrating clean but intermittent power sources such as wind and solar fully into the electric grid. Major advances in batteries will also be needed to significantly expand the electrification of vehicles and thereby move beyond our petroleum-based land transportation system. In general, effective energy storage will be needed to provide the kind of flexible sourcing and delivery of energy that will be essential to diversifying our energy portfolio and moving decisively away from our century-long dependence on fossil fuels.
One of the most promising next-generation battery architectures is thought by many to be the so-called lithium-air (Li-air) battery. Many experts believe that Li-air batteries could potentially revolutionize the transportation sector. The key promise of the Li-air battery lies in its potential for a major increase in "energy density."
Energy density is the amount of energy you can store per volume or per weight (known as "volumetric" or "gravimetric" energy density, respectively). Gasoline has a relatively high energy density. By comparison, the energy density of today's hybrid car batteries is rather low. In automobiles—when respective performance is measured on a tank-to-wheels basis—gasoline is more than ten times as energy dense by weight as today's Li-ion batteries, according to an analysis by researchers from IBM (see second article listed under "Publications" below).
Energy density is important because greater energy density translates directly into longer driving range. The 2013 Tesla Model S has an EPA certified driving range of 265 miles, but thanks largely to the battery load, the car weighs more than 4,500 pounds. It also has a starting price of over $52,000. For electric vehicles to become widely adopted, it's generally thought that the vehicles will need to have a driving range of well over 300 miles with a significantly more compact and lighter battery.
Many experts believe that a Li-air battery might eventually fit the bill. Theoretically, a Li-air battery could have an energy density equal to that of gasoline—and therefore support a driving range on a single charge equivalent to what you can obtain on a tank of gas. But achieving a real Li-air battery with such specifications will mean overcoming a formidable series of scientific and engineering challenges.
With the help of nanoscience, a team of researchers led by Jun Liu of DOE's Pacific Northwest National Laboratory (PNNL)—including collaborators from both PNNL and Princeton University—has recently designed a new electrode that may help to overcome one key such barrier. The work, supported by the DOE Office of Science, was reported in the journal Nano Letters.
 Photo courtesy of Pacific Northwest National Laboratory
Photo courtesy of Pacific Northwest National Laboratory
Jun Liu led a team including researchers from PNNL and Princeton University.
Both the promise and the challenges of the Li-air battery can be traced partly to the peculiar properties of lithium itself. The third element in the periodic table, after hydrogen and helium, lithium is extremely light in weight and also one of the most chemically reactive elements on earth. It readily combines with other molecules—and in the process often gives off electrons that, in turn, can drive an electric circuit. This property makes lithium excellent for battery chemistries.
But the same property can also cause mischief. For example, lithium readily reacts with water and can actually burst into flames when exposed to it. So moisture poses a safety hazard. In addition, lithium can react with a host of other gases, chemicals, and substances in the air, rapidly diminishing battery performance through corrosion. Ironically, these problems raise the question of whether a Li-air battery, its name notwithstanding, could ever run on ambient air—one in a long list of challenges still to be tackled by researchers.
But one key challenge high on the list of many experts has been effectively addressed by Liu's group—that is the tendency of the battery's positive or "air" electrode to "clog" during discharge.
Chemically, in a Li-air battery, lithium reacts with oxygen (O2) to form, ideally, lithium peroxide (Li2O2), and in the process gives off electrons to drive an electric circuit (that's why the system is sometimes also called the "lithium-oxygen" or "Li-O2" battery).
As the lithium discards its electron, positive lithium ions flow from the battery's negative electrode toward a porous, net-like or sieve-like air electrode that is saturated with O2 molecules and a catalyst. The oxygen molecules combine with the lithium ions to form lithium oxide or lithium peroxide. The reaction is reversed on charging.
Technically, in the Li-air battery's architecture, the lithium metal forms the anode of the battery, while the oxygen itself is actually functioning as the battery's cathode. The Li-air architecture is inherently lighter in weight and releases multiple electrons per cycle; both properties translate into improved energy density. In a Li-ion battery, by contrast, both cathode and anode are typically layered compounds, which allow only one positive lithium ion at a time to move between cathode and anode. This largely limits the theoretical capacity of Li-ion batteries.
A major challenge for the Li-air battery, however, has been the tendency of the lithium oxide and lithium peroxide molecules to clog the battery's porous air electrode as these molecules accumulate from the reaction between lithium and oxygen. The clogging progressively blocks the path of the oxygen and thereby leads to rapid deterioration in battery performance and ultimately cessation of discharge.
To overcome this problem, the PNNL team developed a new, nanostructured air electrode. The researchers took sheets of graphene—a special, one-atom thick form of carbon—and immersed them in an oil-water emulsion. The result was a unique three-dimensional porous structure, with pores ranging in size from micro- to nanoscale.
It turned out that the microscale pores, linked by tunnels, provided excellent flow for the O2 molecules, while the nanoscale pores provided excellent sites for the reactions of oxygen and lithium ions (producing mostly lithium peroxide, in this case)—so much so that the electrode operated efficiently without the usual presence of a catalyst. So the new electrode avoided the clogging while at the same time enhancing the Li-O2 reactions.
“The researchers were able to achieve an energy capacity of 15,000 milliamp-hours per gram...the highest value achieved to date for a battery of this type.”
The researchers used transmission electron microscopy at PNNL's Environmental Molecular Science Laboratory to characterize the electrode, and supplemented their understanding of the role of the material in facilitating the lithium oxidization through computational modeling at the National Energy Research Scientific Computing Center at DOE's Lawrence Berkeley National Laboratory.
The researchers were able largely to overcome the clogging problem and to achieve a specific capacity of 15,000 milliamp-hours per gram of carbon (15,000 mAh/g), the highest value achieved to date for a battery of this type tested under similar conditions.
The outcome achieved by Liu's group is clearly a lab-bench result rather than a practical battery design and addresses only one in a long list of challenges that must be overcome before Li-air batteries become practical. But it is a testament to the power of contemporary nanoscience to achieve the kinds of fundamental breakthroughs that will be needed on the path to revolutionary improvements in electricity storage and a host of other energy technologies.
--Patrick Glynn, DOE Office of Science, Patrick.Glynn@science.doe.gov
Publications
Jie Xiao, Donghai Mei, Xiaolin Li, Wu Xu, Deyu Wange, Godon L. Graff, Wendy D. Bennett, Zimin Nie, Laxmikant V. Saraf, Ilhan A. Aksay, Jun Liu, and Ji-Guang Zhang, “Hierarchically Porous Graphene as a Lithium-Air Battery Electrode,” Nano Letters 11, 5071 (2011).
G. Girishkumar, B. McCloskey, A. C. Luntz, S. Swanson, and W. Wilcke, “Lithium-Air Battery: Promise and Challenges,” Journal of Physical Chemistry Letters 1, 2193 (2010).
Funding
Research: DOE Office of Science, Office of Basic Energy Sciences
Facilities: Environmental Molecular Sciences Laboratory: DOE Office of Science, Office of Biological and Environmental Research; National Energy Research Scientific Computing Center: DOE Office of Science, Office of Advanced Scientific Computing Research
Related Links
PNNL Chemical and Materials Science Division
DOE Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division


