Scientists Create World’s Smallest Battery
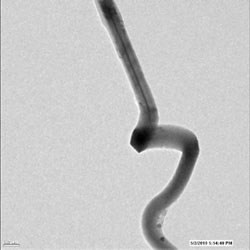
Image shows distortion of nanowire electrode during charging. Researchers were able to observe charging and discharging in real time at atomic-level resolution.
Rechargeable lithium-ion (Li-ion) batteries have become the workhorse of the contemporary electronic age, powering everything from cell phones and laptop computers to hybrid electric vehicles. But while superior to many alternatives for electrical energy storage, Li-ion batteries are not optimal in every respect. Despite much progress over the years, their performance and longevity still fall short of ideal.
Could nanotechnology be harnessed to improve Li-ion battery performance? That is the premise of a University of Maryland-led Energy Frontier Research Center (EFRC), one of 46 such research centers around the Nation established by the DOE Office of Science in 2009 to focus on overcoming basic science challenges on the road to a new energy economy for the Nation.
In their effort to understand the behavior of Li-ion battery components at the nanoscale, researchers at the Center for Integrated Nanotechnologies (CINT)—a scientific user facility located at Sandia and Los Alamos National Laboratories— constructed the world's smallest battery inside the high-vacuum sample chamber of a transmission electron microscope (TEM). The multi-disciplinary team included researchers from the Maryland-led EFRC and CINT users from Pacific Northwest National Laboratory, the University of Pittsburgh, and the University of Pennsylvania.
All batteries are made up of three components—an anode, or negative electrode; a cathode, or positive electrode; and an electrolyte, typically a fluid, providing a conduction pathway for ions between anode and cathode. When a Li-ion battery is charging, Li ions move through the electrolyte from the cathode to the anode. When the battery discharges, the ions flow in the reverse direction.
The aim of researcher Jian Yu Huang of Sandia and his EFRC colleagues was to observe changes in the anode structure during charge/discharge cycles—in real time and at atomic-level resolution. The anode they constructed consisted of a single tin oxide (SnO2) nanowire 100 nanometers in diameter and 10 micrometers long, while a larger diameter rod of lithium cobalt oxide (LiCoO2) served as the cathode.
To construct the battery, Huang and his team had to overcome a key technical challenge: a liquid electrolyte would typically evaporate rapidly in the high vacuum environment of the TEM. Huang was able to overcome this problem by using an extremely low vapor-pressure ionic liquid (liquid salt) as the electrolyte.
Said Huang of the work, reported in the journal Science: "This experiment enables us to study the charging and discharging of a battery in real time and at atomic scale resolution, thus enlarging our understanding of the fundamental mechanisms by which batteries work."
"What motivated our work," added Huang, "is that Li-ion batteries have very important applications, but the low energy and power densities of current Li-ion batteries cannot meet the demand. To improve performance, we wanted to understand Li-ion batteries from the bottom up, and we thought in-situ TEM could bring new insights to the problem."
Battery research groups do use nanomaterials as anodes, but in bunches of wires rather than individually—a process, according to Huang, that resembles "looking at a forest and trying to understand the behavior of an individual tree." The plan of Huang and his team was to use a single "tree" to more closely study its behavior.
An unexpected finding of the researchers was that the tin oxide nanowire nearly doubles in length during charging—far more than its diameter increases—a phenomenon that needs to be considered in constructing batteries so as to avoid short circuits that may shorten battery life. "Manufacturers should take account of this elongation in their battery design," Huang said. (Until now, the common belief of workers in the field has been that batteries swell across their diameter, not longitudinally.)
Huang's group found this flaw by following the progression of the lithium ions as they travel along the nanowire and create what researchers christened the "Medusa front"—an area where a high density of mobile dislocations causes the nanowire to bend and wiggle as the front progresses. The web of dislocations is caused by lithium penetration of the crystalline lattice of the electrode. "These observations prove that nanowires can sustain large stress induced by lithiation without breaking, indicating that nanowires are very good candidates for battery electrodes," said Huang.
"Our observations—which initially surprised us—tell battery researchers how these dislocations are generated, how they evolve during charging, and offer guidance in how to mitigate them," Huang said. "This is the closest view to what's happening during charging of a battery that researchers have achieved so far."
Lithiation-induced volume expansion, plasticity, and pulverization of electrode materials are the major mechanical defects that plague the performance and lifetime of high-capacity anodes in lithium-ion batteries, Huang said. "So our observations of structural kinetics and amorphization [the change from normal crystalline structure] have important implications for high-energy battery design and in mitigating battery failure."
The electronic noise level generated from the researchers' measurement system was too high to read electrical currents, but Sandia co-author John Sullivan estimated a current level of a picoampere flowing in the nanowire during charging and discharging. The nanowire was charged to a potential of about 3.5 volts, Huang said.
A picoampere is a millionth of a microampere. A microampere is a millionth of an ampere. Nonetheless, a picoampere is a substantial current, given the nanoscale dimensions of the battery.
Although the work was carried out using tin oxide (SnO2) nanowires, the experiments can be extended to other materials systems, either for cathode or anode studies, Huang said. Because nanowire-based materials in Li-ion batteries offer the potential for significant improvements in power and energy density over bulk electrodes, more stringent investigations of their operating properties should improve new generations of plug-in hybrid electric vehicles, laptops and cell phones.
"The methodology that we developed should stimulate extensive real-time studies of the microscopic processes in batteries and lead to a more complete understanding of the mechanisms governing battery performance and reliability," he said. "Our experiments also lay a foundation for in-situ studies of electrochemical reactions, and will have broad impact in energy storage, corrosion, electrodeposition, and general chemical synthesis research."
Other researchers contributing to this work include Xiao Hua Liu, Nicholas Hudak, Arunkumar Subramanian and Hong You Fan, all of Sandia; Li Zhong, Scott Mao and Li Qiang Zhang of the University of Pittsburgh; Liang Qi, Akihiro Kushima and Ju Li of the University of Pennsylvania; and Chong Min Wang and Wu Xu of Pacific Northwest National Laboratory (PNNL), who also contributed to the characterization of the anode using instrumentation at PNNL's Environmental Molecular Science Laboratory.
Funding
Research: Sandia National Laboratories Laboratory Directed Research and Development funds; DOE Office of Science, Office of Basic Energy Sciences through the Center for Science of Precision Multifunctional Nanostructures for Electrical Energy Storage, a DOE Energy Frontier Research Center (EFRC) led by the University of Maryland; and DOE Office of Science, Office of Biological and Environmental Research
Facilities: Center for Integrated Nanotechnologies: DOE Office of Science, Office of Basic Energy Sciences; Environmental Molecular Research Laboratory: DOE Office of Science, Office of Biological and Environmental Research
Publication
J. Y. Huang et al. "In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode," Science 330 1515 (2010)
Related Links
World's Smallest Battery Charging in Real Time - YouTube or 2 MB WMV File


