Profiting from Waste?
 Photo courtesy of the National Archives and Records Administration
Photo courtesy of the National Archives and Records Administration
Thermoelectric materials offer a means of generating electricity from heat.
Roughly two-thirds of the energy we use in the world today ends up as waste heat—whether it's escaping through the smokestack of a power plant or the tailpipe of your automobile — which is why the idea of recycling waste heat and transforming it back into useful energy remains an enticing prospect. That prospect partly explains the longstanding interest in thermoelectric materials—semiconductors that convert heat into electricity.
Scientists and engineers have worked for decades on these materials, and some limited applications have been found for them. NASA's Mars rover Curiosity, for example, is powered by a thermoelectric generator, which produces electricity from heat emanating from decaying radioactive material in a chamber inside the vehicle. Some automotive firms are looking into the idea of generating electricity from tailpipe exhaust or engine heat. And a DOE Office of Science-supported team from MIT recently devised an impressive new solar cell based on a thermoelectric device (see "More Heat than Light").
Until recently, however, thermoelectric devices have remained mostly a niche technology. That's largely because thermoelectric materials have comparatively low energy conversion efficiencies—normally converting only about 5 to 7 percent of heat energy into electricity (as compared to, say, 20 percent efficiency for silicon wafer solar cells generating electricity from sunlight).
But now a team of researchers led by a DOE Energy Frontier Research Center (EFRC) has fabricated a thermoelectric material with a conversion efficiency of roughly 15 percent. This record-breaking result could be an important step toward making thermoelectric devices viable as an energy-recycling technology capable of deployment on a wider scale. The work was reported in the journal Nature.
The new material fabricated by the researchers converts heat to electricity "at the highest possible efficiency," according to Mercouri G. Kanatzidis, Charles E. and Emma M. Morrison Professor of Chemistry at Northwestern University, who led the research team.
"At this level," said Kanatzidis, "there are realistic prospects for recovering high-temperature waste heat and turning it into useful energy."
“At this level, there are realistic prospects for recovering high-temperature waste heat and turning it into useful energy.”
Mercouri G. Kanatzidis
Kanatzidis is a principal investigator in the Center for Revolutionary Materials for Solid State Energy Conversion, an EFRC led by Michigan State University, in which Northwestern University is a partner. It is one of 46 such centers established by the DOE Office of Science around the country in 2009 to accelerate basic research on energy.
Kanatzidis and his team relied on a well-established strategy for improving thermoelectric performance but ingeniously extended the strategy in a way that broke through a longstanding barrier. They more than doubled what is known as the thermoelectric "figure of merit."
Figure of merit is a number used by scientists and engineers to quantify the relative efficacy of a material in achieving a particular purpose. The quantities and terms for figure of merit vary depending on the category of material. The actual mathematics of the thermoelectric figure of merit is a bit complicated, but it essentially depends on a ratio of electrical conductivity to heat conductivity. To get a good thermoelectric effect, you basically want a material that is a relatively good conductor of electricity and a relatively poor conductor of heat. That is, when electrical conductivity is relatively high and heat conductivity is relatively low, you have a high figure of merit. For most of the past few decades, the figure of merit for thermoelectric materials has hovered around 1.
The main strategy for improving these materials—and thereby increasing their figure of merit—has been to reduce their heat conductivity while maintaining, or possibly increasing, their electrical conductivity. And the main tactic for achieving this result is called "nanostructuring."
Heat propagates through a material in the form of quasi-particles called "phonons," which are a quantum-mechanical name for vibrations of atoms. Back in the mid-1990s, it was discovered that by introducing tiny (i.e., nano-size) gaps and/or irregularities in a thermoelectric material, one could disrupt the propagation of phonons and thereby suppress the conduction of heat. It would be a bit like digging a series of ditches or erecting barriers such as barbed wire in a field to slow an advancing army.
Using this tactic, previous researchers had been able to achieve figure of merit in the range of 1.5 to 1.8. However, prior to the work of Kanatzidis's EFRC team, performance fell short of what has been the longstanding goal of this research—namely, a figure of merit of 2 or greater.
The reason? It turns out that while the nanostructures disrupted the path of most phonons, about 20 percent of phonons—those with long so-called "mean free paths"—were slipping by the barriers. These phonons, in effect, were operating on a slightly larger "micro" scale rather than on a "nano" scale and were consequently mostly unaffected by the tiny nanoscale defects and/or gaps.
To disrupt the greatest possible percentage of phonons, Kanatzidis and his team implemented a three-tiered strategy, successively aimed at disrupting the propagation of phonons with short, medium, and long mean free paths.
Their work focused on the alloy lead telluride (PbTe)—the most effective known thermoelectric material at very high temperatures (750-900 degrees Kelvin, or about 890 to 1160 degrees Fahrenheit).
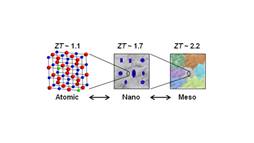 Image courtesy of Mercouri G. Kanatzidis.
Image courtesy of Mercouri G. Kanatzidis.
Successive texturing of the material raised the figure of merit ("ZT") from 1.1 to 2.2.
First, to address the short category, the researchers "doped" the lead telluride with sodium, scattering a relatively small quantity of sodium atoms irregularly through the alloy's crystal lattice.
This "doping," or introduction of impurities into a material, is standard in the fabrication of semiconductors and was designed to increase the electrical conductivity of the lead telluride—while at the same time creating defects that would act as barriers to the propagation of short mean-free-path phonons.
Second, to address the medium category, the researchers embedded somewhat larger nanocrystals of strontium telluride (SrTe) in the material by a process known as endotaxy, aimed at disrupting the propagation of medium mean-free-path phonons.
These latter two steps addressed phonons with nanoscale mean free paths.
The third step, and the coup de grâce, was to introduce microscale defects by breaking up the material and reassembling it using a process called "spark plasma sintering." The process introduced microscopic cracks throughout the material, which functioned to disrupt phonons with the long, or microscale, mean free paths.
By curbing the propagation of all three categories of phonons, the combination of the three steps substantially reduced the material's heat conductivity. The steps also had the collateral benefit of increasing the material's electrical conductivity.
The researchers analyzed the structure of the new material using both transmission electron microscopy and a technique called three-dimensional atom probe tomography. They also measured electrical conductivity and measured and estimated heat conductivity. They compared the textured, sintered material with ingots of the same material that lacked the microscale defects that resulted from the third step. The performance of the sintered material was substantially better than that of the less textured ingots.
In the end, the researchers were able to achieve a record-breaking figure of merit for the sintered material of 2.2 at a temperature of 915 degrees Kelvin (approximately 1187 degrees Fahrenheit).
While the experimental material is not quite ready for commercial use, the researchers say they have hit upon a generally applicable technique that has not only broken the "2" barrier but that should also eventually help enable production of high-efficiency, commercially viable thermoelectric devices.
"Improving the figure of merit never stops—the higher the figure of merit the better," Kanatzidis said. "We would like to design even better materials and reach 2.5 or 3. We now have what we call a panoscopic approach to the challenge—that is, we are tackling it at all length scales. We continue to have new ideas and are working to better understand the materials we have."
—Patrick Glynn, DOE Office of Science, Patrick.Glynn@science.doe.gov
Research Funding
DOE Office of Science, Office of Basic Energy Sciences
Publications
Kanishka Biswas, Jiaquing He, Ivan D. Blum, Chun-I Wu, Timothy P. Hogan, David N. Seidman, Vinayak P. Dravid, and Mercouri G. Kanatzidis, "High-performance bulk thermoelectrics with all-scale hierarchical architectures," Nature 489, 414 (2012).
Geoff Brumfield, "Out of disorder comes electricity: Nanostructured thermoelectric material breaks record for turning heat into electricity," Nature News, September 19, 2012, at http://www.nature.com/news/out-of-disorder-comes-electricity-1.11445.
Fabio Pullizzi, "Thermoelectrics: The panoscopic approach," Nature Nanotechnology 7, 622 (2012).
Related Links
Center for Revolutionary Materials for Solid State Energy Conversion

