Beating Nature at her Own Game?
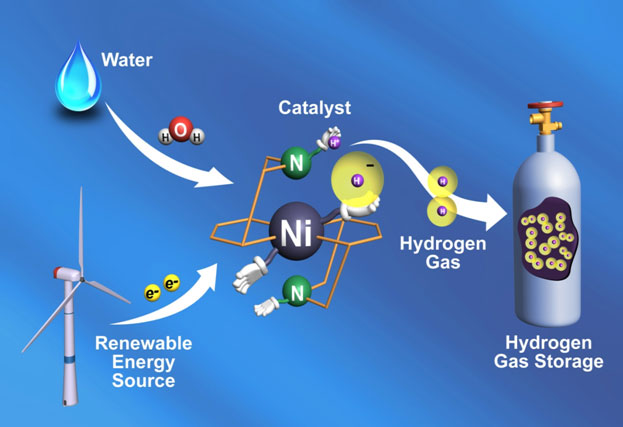 Image courtesy of Pacific Northwest National Laboratory
Image courtesy of Pacific Northwest National Laboratory
Systemic view of how catalyst might fit into a renewable energy production and storage system.
Major improvements in our ability to store electrical energy will be essential if we are to deploy renewable energy on any significant scale. For example, before renewable sources such as wind and solar can be effectively integrated into the nation's electrical grid, we must be able to stabilize their intermittent output. That means storing energy when the sun and wind provide it, and releasing the stored energy when the sun is no longer shining and the wind has died down. An effective system for accomplishing this will require storage capabilities that lie beyond current technologies. And this instance represents only one of many areas where greatly improved electrical energy storage will be critical to the development and management of a clean energy economy.
That is why scientists are working at the very frontiers of chemistry, biology, applied physics, and materials science today in an effort to understand and radically improve electrical energy storage capabilities by manipulating matter at the atomic and molecular levels. Now researchers at the U.S. Department of Energy's Pacific Northwest National Laboratory (PNNL), taking a page from Nature's playbook, have developed a new catalyst that enables storage of energy through the conversion of electrical energy to hydrogen fuel—at a rate ten times faster than Nature has been able to accomplish this. The work is reported in the journal Science.
“We want to store energy as densely as possible. Chemical bonds can store a huge amount of energy in a small amount of physical space.”
Morris Bullock
Pacific Northwest National Laboratory
The research—led by the PNNL-based Center for Molecular Electrocatalysis, one of 46 Energy Frontier Research Centers established by the DOE Office of Science in 2009—is pointing the way toward new kinds of catalysts that could help make hydrogen-based fuel cells more economical and practical as a means of both storing and generating electrical energy.
Rechargeable batteries, such as lithium-ion batteries, store electrical energy in the form of electrons. Another way of storing electrical energy is to convert it to chemical energy. That is what the PNNL researchers set out to do—to convert electrical energy to the energy embodied in the chemical bonds that hold hydrogen molecules (H2) together—energy ready to be released again when the hydrogen is oxidized to produce water.
"We want to store energy as densely as possible," said PNNL's Morris Bullock, who led the study. "Chemical bonds can store a huge amount of energy in a small amount of physical space."
Instead of focusing on batteries, therefore, the PNNL group worked with the basic technology of fuel cells. In a basic fuel cell, hydrogen combines with oxygen to produce electricity and water. The reaction is also reversible. If you pass an electrical current through water, you will cleave some of the H2O molecules to produce oxygen and hydrogen gases. For efficiency, however, both reactions require a catalyst. To date platinum has proved to be the most effective such catalyst, but of course it is both rare and prohibitively expensive.
There are, however, common microbes that can also produce hydrogen gas from water. They do so using an organic catalyst, a protein or enzyme known as a "hydrogenase." These enzymes also incorporate metal, but typically in the form of nickel and iron, which are both more abundant and of course much cheaper than platinum. Could we use the enzyme itself as a catalyst for an energy storage system? It turns out that these enzymes are difficult to extract and produce in quantity, and they are not terribly stable or survivable outside the specific natural environments to which they are accustomed. So the naturally occurring enzymes would not really be suited for an industrial application.
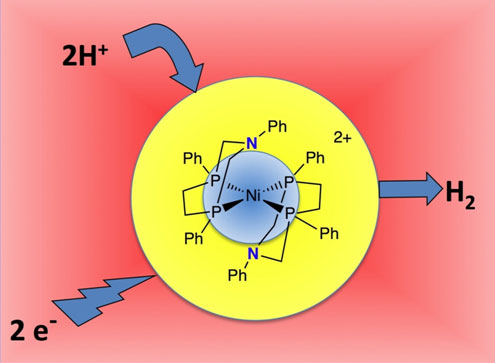 Image courtesy of Pacific Northwest National Laboratory
Image courtesy of Pacific Northwest National Laboratory
Schematic showing catalyst operation.
Rather than use the actual hydrogenase, the PNNL researchers set out to build their own synthetic chemical catalyst by mimicking the enzyme's "design." The team focused specifically on the "active site" of the enzyme, or the part of the enzyme that actually performs the catalytic reaction. In doing so they drew on extensive previous studies of hydrogenases. Through a technique called protein film voltammetry, the catalytic action of these enzymes had been extensively described, while studies involving protein crystallography provided insight into the structure of the active site.
Essentially, the active site of the hydrogenase consists of a metal atom at the hub of several molecular "spokes" (technically called "ligands") to which organic compounds known as amines are appended. One feature of amines—organic molecules built around a nitrogen atom—is their strong tendency to bond with protons.
Since a hydrogen atom consists of one proton and one electron, you can make a positively charged hydrogen ion (H+) by removing the electron and a negatively charged hydrogen ion (H-) by adding a second electron. In other words, a positively charged hydrogen ion and a proton are one and the same. A negatively charged hydrogen ion is sometimes called a "hydride."
In the catalytic action of the hydrogenase, the amines function as what are called "proton relays." They essentially grab a proton or H+ from an H2O molecule and then combine it with an H-—like a relay runner grabbing a baton from her predecessor and then passing it on to her successor. Together the H+ and H- combine to form a molecule of hydrogen gas, or H2. The amines work in tandem with the metal atom (nickel, in the case of the catalyst built by the researchers). While the amine grabs one proton, the nickel atom adds two electrons to the second proton, making it a hydride. As a result, the two hydrogen atoms end up with opposite charges—the one grabbed by the amine is positive, while the one held by the nickel is negative. The opposite electrical charges of the two hydrogen atoms cause them to mutually attract and ultimately to bond together as hydrogen gas, or H2.
In their experiment, the researchers used a special proton-rich solvent in place of water. To energize the reaction, they ran an electric current through the solvent. After many tweaks to the synthetic design, they built a catalyst capable of creating hydrogen gas at the rate of 33,000 molecules per second. Adding water to the solution brought the rate up to over 100,000 molecules per second. This compares to the reported rate for natural hydrogenases—hydrogenases as they occur in actual microbes—of 9,000 molecules per second. So the synthetic version appears roughly ten times as fast as Nature's.
The new system is impressive, though not quite ready for prime time. For one thing, the experimental system requires a higher voltage than would be optimal in an industrial process. But the research lays open a path to the rational design of ever more effective catalysts, using Nature's blueprint as a model to build and improve on. And it shows how our ever deeper and more detailed understanding of matter and processes at the atomic and molecular levels hold the key to revolutionizing our energy economy, enabling us to develop the breakthrough technologies ultimately needed to reduce our dependence on imported oil and fossil fuels.
—Patrick Glynn, DOE Office of Science, Patrick.Glynn@science.doe.gov
Funding
DOE Office of Science, Office of Basic Energy Sciences
Publication
M L Helm et al., "A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s-1 for H2 production," Science 333, 863 (2011)

